Home
Subscription
February 2026 issue
January 2026 issue
BACK ISSUES
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
2015
2014
2013
2012
2011
2010
2009
2008
2007
2006
|
||
|
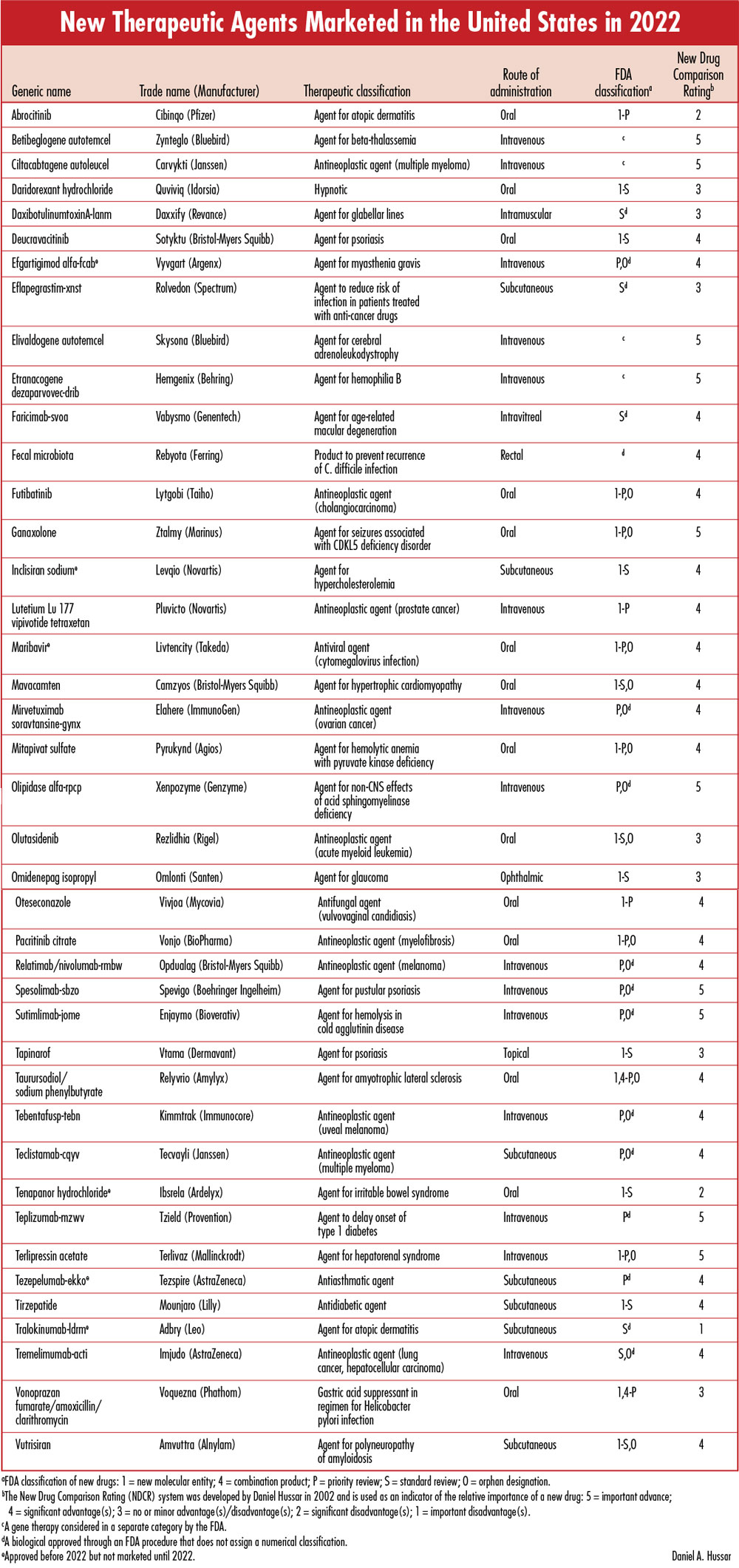
Each issue will include an editorial on a topic that is important for the profession of pharmacy, as well as a review of a new drug that includes a comparison of the new drug with previously marketed drugs that are most similar in activity, and a New Drug Comparison Rating (NDCR) for the new drug. Read on for this month's issue. May 2023 Issue [Download PDF format] In this issue: • Mifepristone and the FDA's Responsibility: Lessons from Dr. Kelsey and Thalidomide • New Therapeutic Agents Marketed in the United States in 2022 |
||
EDITORIAL:Mifepristone and the FDA's Responsibility: Lessons from Dr. Kelsey and ThalidomideThe mission of the Food and Drug Administration is to protect and promote the public health. An acclaimed example of FDA's commitment to its mission is described in an FDA commentary titled, "Frances Oldham Kelsey: Medical reviewer famous for averting a public health tragedy." The commentary begins: "Frances Oldham Kelsey, recipient of the highest recognition attainable for a U.S. civil servant for her role in saving perhaps thousands from death or long-term incapacitation…" Other excerpts from this commentary follow:
"Their (FDA medical officers) principal duty was to review new drug applications, a legal requirement in which manufacturers had to provide evidence of a drug's safety before it could go on the market. One of the first applications she was assigned was for thalidomide, which was already available in dozens of countries around the world. Dr. Kelsey, despite constant pressure from the company, refused to approve the application because of its inadequate evidence." "Dr. Kelsey adamantly insisted on scientifically reliable evidence, which she felt the application sorely lacked. Approximately a year later researchers in Germany and Australia linked thalidomide to clusters of rare, severe birth defects – hands and feet projecting directly from the shoulders and hips – that eventually were shown to involve thousands of babies." "Her (Dr. Kelsey's) contributions have been widely recognized through Presidential and other awards, honorary academic degrees, and educational facilities named after her. Also, in October 2000 Dr. Kelsey was inducted into the National Women's Hall of Fame, and in 2010 Commissioner Hamburg conferred the first Dr. Frances O. Kelsey Award for Excellence and Courage in Protecting Public Health on Dr. Kelsey herself." It was in the early 1960s that Dr. Kelsey's concerns prevented the approval and marketing of thalidomide in the U.S., and any further study of the drug was abandoned for many years. It is noteworthy that, notwithstanding thalidomide's teratogenicity and infamy, it was studied and approved many years later in 1998, initially for the treatment of acute cutaneous complications of leprosy and subsequently for the treatment of multiple myeloma. Analogs of thalidomide, lenalidomide (e.g., Revlimid) and pomalidomide (Pomalyst), are more commonly used in the current treatment of multiple myeloma and certain other conditions, but all three agents are contraindicated during pregnancy, include a boxed warning in their labeling about severe life-threatening birth defects, and are available only through a restricted Risk Evaluation and Mitigation Strategy (REMS) program. Dr. Kelsey's accomplishments were numerous but her single greatest accomplishment was preventing thalidomide from being approved in the U.S. because there was not sufficient documentation of its safety. The FDA's subsequent recognition of her and the establishment of an award in her honor for excellence and courage in protecting public health confirms the FDA's commitment to protect the public health AND that the public health includes the lives of the unborn, as well as the born. Additional confirmation that the FDA's responsibility includes the protection of unborn babies is evident by the inclusion of a section (8.1) in the product labeling (package insert) of all prescription medications regarding risks of the medication if used during pregnancy. Although some of this information may pertain to risks of the medication for the woman taking the medication (e.g., risk of miscarriage), the large majority of the information describes possible risks for the unborn baby. Approval of mifepristoneOn September 28, 2000, the FDA approved mifepristone (Mifeprex, the "abortion pill") for oral use in a regimen with misoprostol for the medical termination of intrauterine pregnancy during the first seven weeks of pregnancy (subsequently changed to "through 70 days gestation"). Some have voiced strong opposition to this decision and some have voiced strong support, but the argumentative debate that has ensued over the years has failed to address the most basic questions. How can this decision to approve mifepristone to cause abortion be reconciled with the FDA's stated mission and responsibility for protecting the public health (including that of unborn babies)? How can this FDA decision be reconciled with FDA's praise and establishment of an award in tribute to Dr. Kelsey's "saving perhaps thousands (of unborn babies) from death or life-long incapacitation?" The short answer is that these decisions/actions CAN'T be reconciled!In the consideration of a new drug application (NDA), the FDA's priorities are to evaluate its effectiveness and safety. The NDA for mifepristone did not request approval for the treatment of illnesses or other medical problems of the women for whom it is prescribed. Rather, approval was requested to terminate pregnancy (i.e., the life of the unborn baby), and there is no question that the drug has a potent abortifacient action for which the manufacturer of Mifeprex states "is 97% effective in terminating early pregnancy." With respect to the safety of mifepristone, discussion has revolved almost entirely about its safety in the women who are prescribed the medication. There has been ongoing debate about its safety in women but this discussion ignores what should be the most important concern. As a consequence of mifepristone's termination of almost 100% of the pregnancies of the women for whom it is prescribed, it is not only unsafe but causes the death of the unborn baby. The FDA's decision to approve mifepristone and the strong differences of opinion regarding abortion have resulted in numerous lawsuits at the district and appellate levels, and eventually at the Supreme Court of the United States (SCOTUS). The 2022 Dobbs v. Jackson ruling of SCOTUS had the effect of overturning the Roe v. Wade decision made by SCOTUS in 1973. The Dobbs decision does not ban, restrict, or enable abortion, but rather provides the authority for voters and legislators in individual states, instead of the federal government, to make pertinent decisions and laws. The Dobbs decision has triggered a cascade of lawsuits that have brought the issue of the FDA approval of mifepristone back to SCOTUS. Proponents of abortion rights and some others assert that justices who are not scientists or health professionals are not in a position to rule on the appropriateness of approving a drug, and that doing so would undermine the authority of the FDA to approve and regulate the use of drugs. However, neither SCOTUS nor the lower courts have requested or otherwise encouraged the filing of these lawsuits. Rather, the lawsuits have been filed because of the failures of the public and legislatures to determine compromises and decisions that are acceptable to most. With respect to the situation that will soon be heard by SCOTUS, the justices do not need scientific or clinical expertise regarding mifepristone. Their decision should be based on whether the FDA fulfilled or failed its mission and responsibility to protect public health by its approval of mifepristone to terminate pregnancy. It is my expectation that SCOTUS will rule that the FDA failed in its responsibility and that the decision to approve mifepristone to terminate pregnancy should be rescinded. Yes, protests and chaos will persist at the national and state levels if this decision, or probably any other decision, is made by SCOTUS. However, the reasons for protest and chaos are less important than actions that result in the death of unborn babies. If SCOTUS rules that the approval of mifepristone to terminate pregnancy should be rescinded, there should be a reduction in the number of abortions for the reason that some women may reconsider their decision during the delay to identify another abortion alternative. The strongest opponents of abortion believe there can be no compromise in a matter with life or death consequences. The strongest proponents for abortion rights believe there should be no restrictions on abortions, or possibly even the termination of infants. Opinion polls indicate that most Americans support abortion rights but with some limitations. We can't expect SCOTUS to render a Solomonic ruling that will resolve the differences of opinion. However, we can hope that its ruling will provide the impetus for those with even strong positions to strive for a détente. Daniel A. Hussar
DanH@pharmacistactivist.com |
||
| ||